What Does Avogadros Law Say About a Gas at Stp
Standard temperature and pressure STP is defined as 0C 27315 K and 1 atm pressure. All matter is mostly empty space.
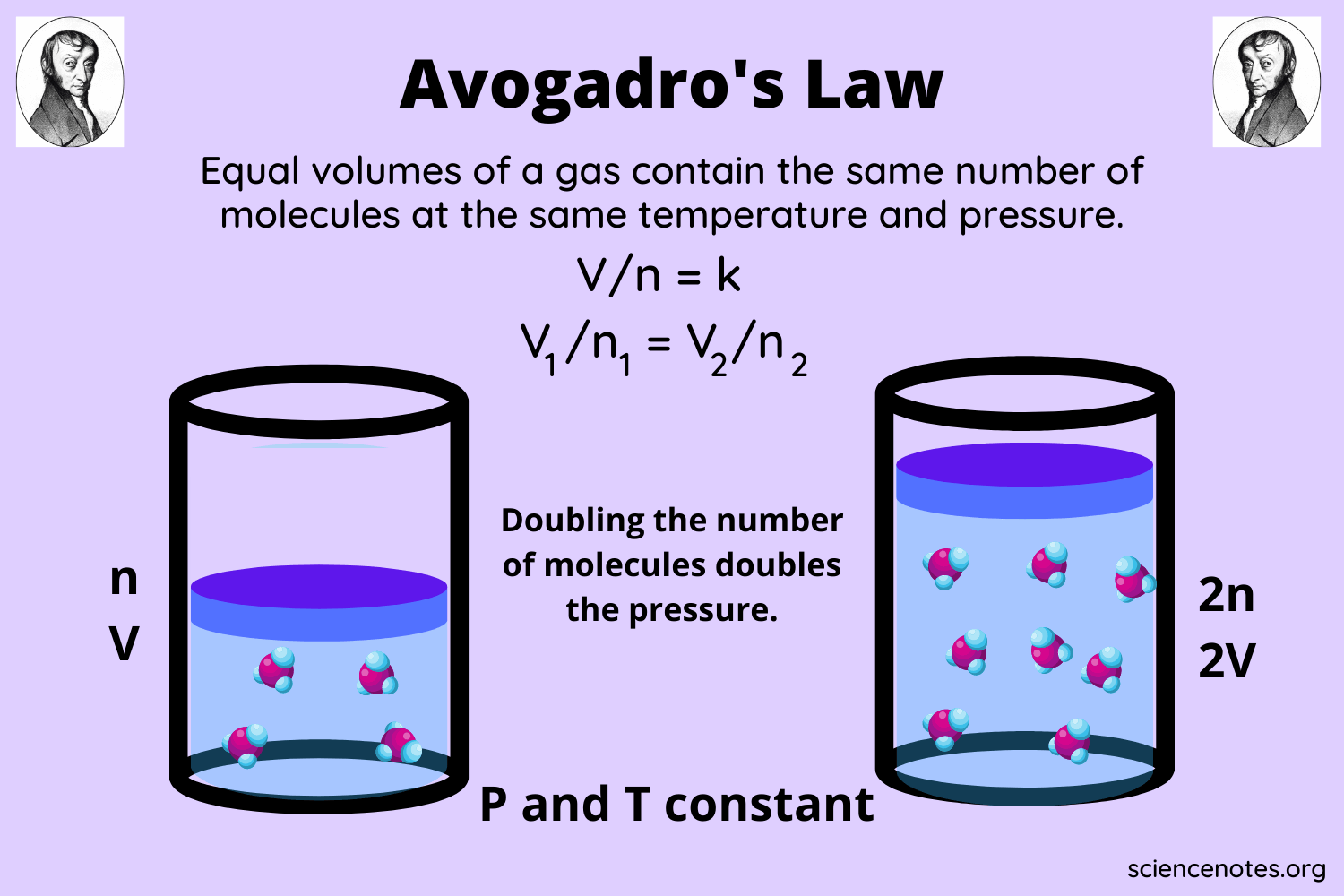
Avogadro S Hypothesis Study Guide Inspirit
What is Avogadros Gas Law.
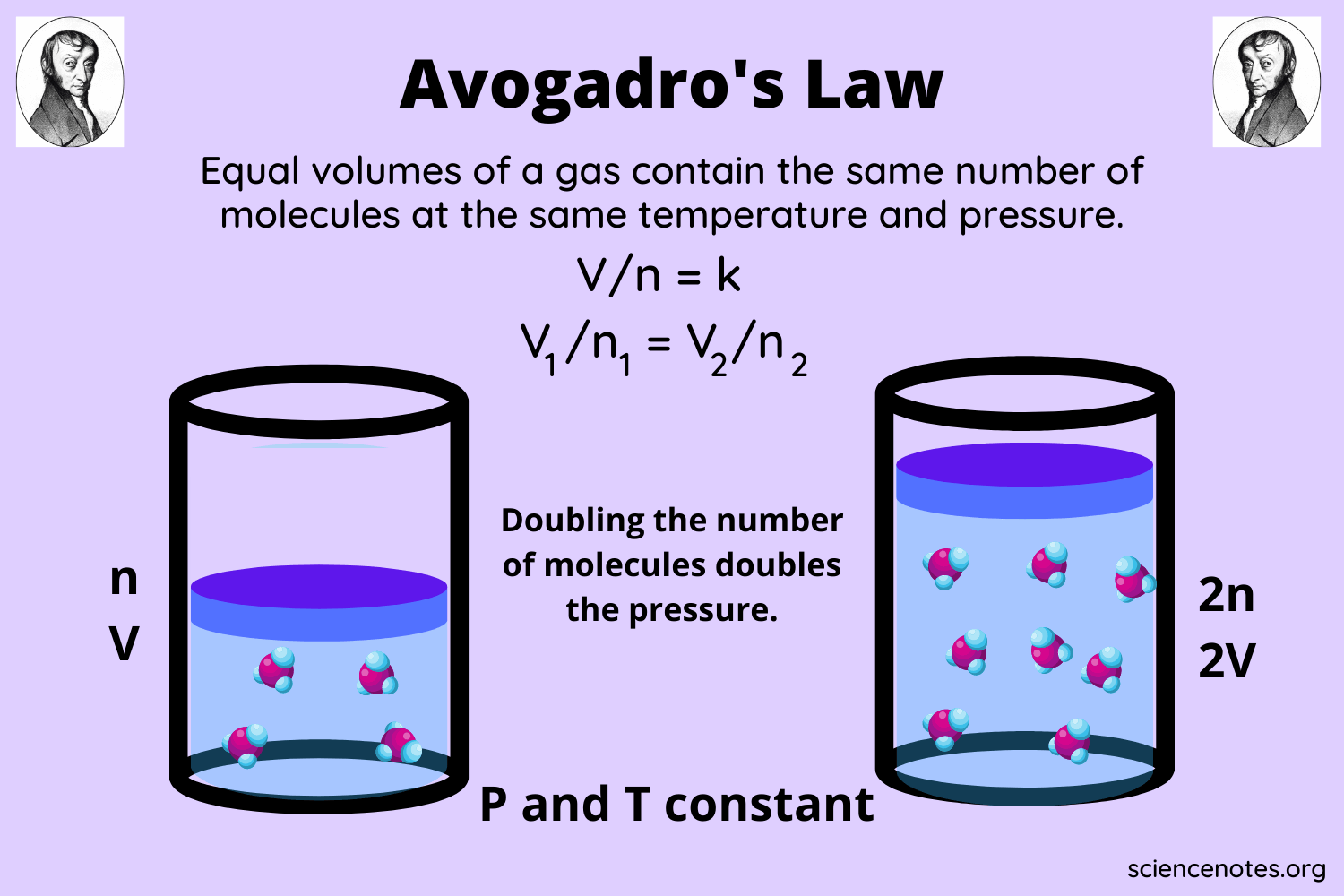
. What does Avogadros law say about stp. What does Avogadros law say about a gas at STP. If one gas variable V or n changes in value either up or down the other variable will also change in the same direction.
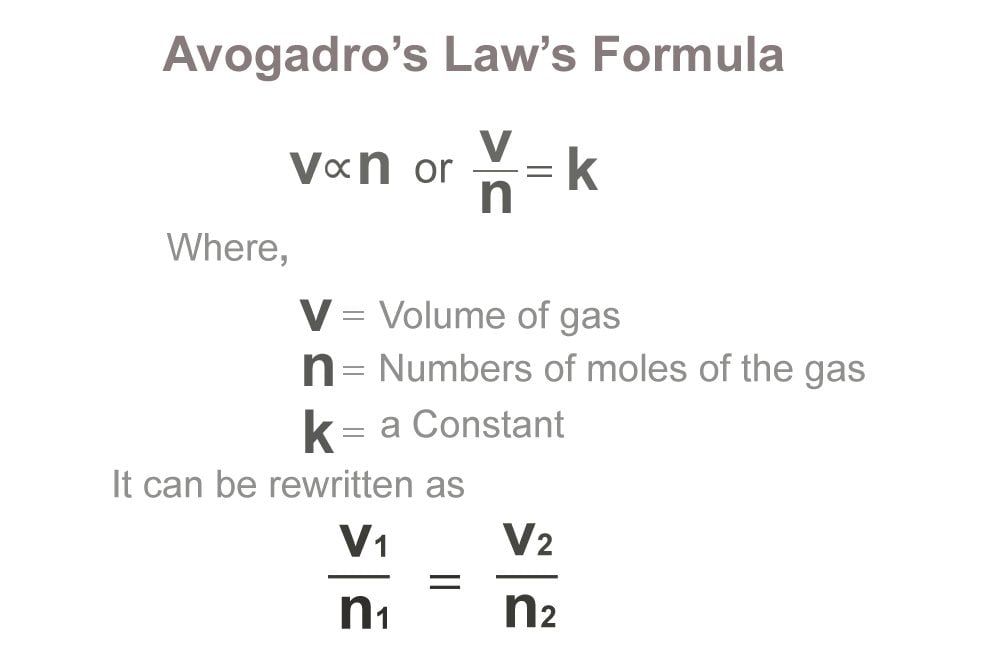
But I was wondering how can this be. V 1 n 2 V 2 n 1. The constants in this relationship would be the temperature t and pressure p The equation for this law is.
According to avogadros law 1 mole of every substance occupies 224 L at STP and contains avogadros number of particles. What does Avogadros law say about at stp. Avogadros Law is stated mathematically as.
The constant K will remain the same value. It occupies 224 L What is the characteristic of 1 mole of. According to avogadros law what is characteristic of 1 mole of gas at STP.
1 mole of gas at STP occupies 224 liters. Now for any gas 1 mole means the exact same thing. 1 mole of any gas has a volume.
STP corresponds with 1 atm pressure 760 torr and 0 o C. P pressure of gas 1 atm at STP V Volume of gas. Facts about Avogadros law One dm 3 of every gas at STP will have molecules 602102322424 268 x 10 23 molecules.
1 mole of gas at STP occupies 224 liters. 224 L of something in the gas phase at STP 1 mol is correct because 1 We dont take into consideration molar mass of the gas we look at the NUMBER OF MOLES. Avogadros Law is the relation which states that at the same temperature and pressure equal volumes of all gases contain the same number of molecules.
Confusion about Avogadros Law and the molar volume of gases So as I remember it from AP Chemistry if it matters as per Avogadros law all ideal gases at STP are supposed to have a molar volume of 224 Lmol exactly. How do you say 602X1023 in plain form. Here k is a proportionality constant V is the volume of a gas and n is the number of moles of a gas.
Equal volumes of gases at the same temperature and pressure have an equal number of molecules. Answer 1 of 3. Avogadros law a statement that under the same conditions of temperature and pressure equal volumes of different gases contain an equal number of molecules.
Avogadros law investigates the relationship between the amount of gas n and volume v. In 1 mole of CH4 there are 6022 1023 CH4 molecules and so on. Avogadros hypothesis states that equal volumes of all gases at the same temperature and pressure contain equal numbers of particles.
For example 100 L of N 2 gas and 100 L of Cl 2 gas contain the same number of molecules at Standard Temperature and Pressure STP. What does Avogadros law say about gas. N number of moles 1.
It may be stated. The Avogadro law is. What does avogadros law say about a gas at STP.
The difference in mass is the mass of the gas itself. What does avogadros law say about a gas at STP. Avogadros law also means the ideal gas constant is the same value for all gases so.
Avogadros Law Equation There are a few ways to write this gas law which is a mathematical relation. It occupies 224 L What is the characteristic of 1 mole of gas at stp. 1 mole of an ideal gas at STP occupies 224 liters.
1 mole of any gas has a volume of 224 at STP. The molar volume of a gas is the volume of one mole of a gas at STP. According to avogadros law 1 mole of every substance occupies 224 L at STP and contains avogadros number of particles.
Constant p 1 V 1 T 1. Its avogadros number How many liters do one mole of any gas occupy at. If its O2 then in mole O2 there are 6022 1023 O2 molecules.
Even in a solid. Avogadros Law Amadeo Avogadro was an Italian physicist who stated in 1811 that the volume of any gas is proportional to the number of molecules of gas measured in Moles symbol mol. Its avogadros number How many liters do one mole of any gas occupy at stp.
Its a direct relationship meaning the volume of a gas is directly propotional to the number of moles the gas sample present. The volume of the bulb is 5000 mL. V n k V n k V is the volume of the gas n is the number of moles of the gas and k.
The law is approximately valid for real gases at sufficiently low pressures and high temperatures. If temperature and pressure are changed equally for all gases each gas will have same 2681023 molecules. According to ideal gas equation.
According to avogadros law what is characteristic of 1 mole of gas at STP. The law was described by Italian chemist and physicist Amedeo Avogadro in 1811. The law is.
How do you say 602X1023 in plain form. Avogadros law also known as Avogadros principle or Avogadros hypothesis is a gas law which states that the total number of atomsmolecules of a gas ie. The amount of gaseous substance is directly proportional to the volume occupied by the gas at constant temperature and pressure.
This empirical relation can be derived from the kinetic theory of gases under the assumption of a perfect ideal gas. In a gas at typical pressures and temperatures the molecular size is so much smaller than the distance between molecules mean free path that it just doesnt matter much. Avogadros Law is a direct mathematical relationship.
The bulb will be weighed when evacuated and when filled with a gas at STP. R gas constant T temperature at STP Thus 1 mole of every gas occupies 224 L at STP.

Avogadro S Law Equal Volumes Of Different Gases At The Same Temperature And Pressure Have The Same Number Of Moles Example Cl2 G H2 G Ppt Video Online Download
Avogadro S Law Definition Formula Equation And Examples

Avogadro S Law Explanation In Terms Of Gaseous State Qs Study

No comments for "What Does Avogadros Law Say About a Gas at Stp"
Post a Comment